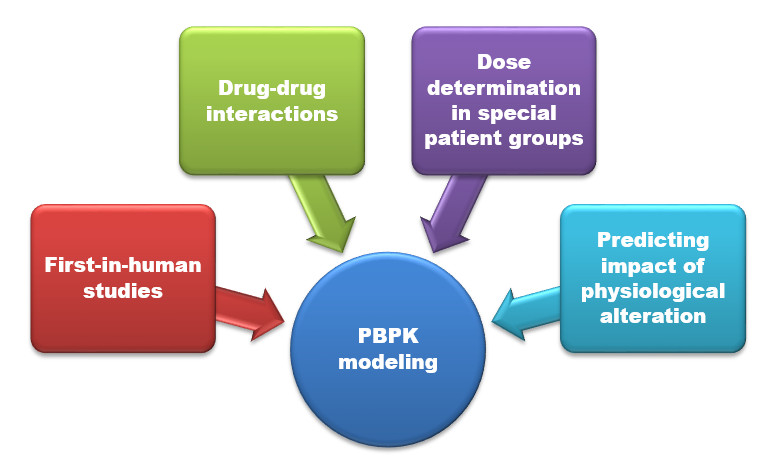
Regulatory Perspective of Physiology based Pharmacokinetic (PBPK) Modeling
Introduction A molecule’s journey from its discovery cradle to the patient’s bedside is highly uncertain and often has a low probability of success. Substantiation of its safety and efficacy to cater to an unmet medical need involves a long span of time and investment

