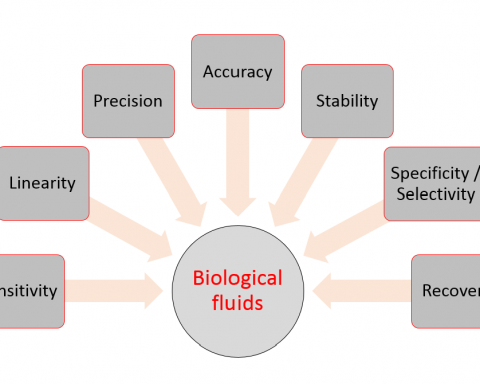
Bioavailability means the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action. For drug products that are not intended to be absorbed into the bloodstream, bioavailability may be assessed by measurements intended to reflect the rate and extent to which the active ingredient or active moiety becomes available at the site of action.
Related: Purpose of Bioavailability Studies
Bioavailability studies are performed for both approved active drug ingredients and therapeutic moieties not yet approved for marketing by the FDA. New formulations of active drug ingredients must be approved by the FDA before marketing. In approving a drug product for marketing, the FDA ensures that the drug product is safe and effective for its labeled indications for use. Moreover, the drug product must meet all applicable standards of identity, strength, quality, and purity. To ensure that these standards are met, the FDA requires bioavailability/pharmacokinetic studies and, where necessary, bioequivalence studies for all drug products (FDA Guidance for Industry, 2003). Bioavailability may be considered as one aspect of drug product quality that links in-vivo performance of the drug product used in clinical trials to studies demonstrating evidence of safety and efficacy.
For unmarketed drugs that do not have full NDA approval by the FDA, in-vitro and/or in-vivo bioequivalence studies must be performed on the drug formulation proposed for marketing as a generic drug product.
[wp_ad_camp_1]
Furthermore, the essential pharmacokinetics of the active drug ingredient or therapeutic moiety must be characterized. Essential pharmacokinetic parameters, including the rate and extent of systemic absorption, elimination half-life, and rates of excretion and metabolism, should be established after single- and multiple-dose administration. Data from these in-vivo bioavailability studies are important to establish recommended dosage regimens and to support drug labeling.
In-vivo bioavailability studies are also performed for new formulations of active drug ingredients or therapeutic moieties that have full NDA approval and are approved for marketing. The purpose of these studies is to determine the bioavailability and to characterize the pharmacokinetics of the new formulation, new dosage form, or new salt or ester relative to a reference formulation.
In summary, clinical studies are useful in determining the safety and efficacy of drug products. Bioavailability studies are used to define the effect of changes in the physicochemical properties of the drug substance and the effect of the drug product (dosage form) on the pharmacokinetics of the drug. Bioequivalence studies are used to compare the bioavailability of the same drug (same salt or ester) from various drug products. Bioavailability and bioequivalence can also be considered as performance measures of the drug product in-vivo. If the drug products are bioequivalent and therapeutically equivalent (as defined above), then the clinical efficacy and the safety profile of these drug products are assumed to be similar and may be substituted for each other.