The U.S. Food and Drug Administration (FDA) is the government agency that regulates marketed drug products and approves marketing of new drug products. The FDA defines a drug product as a finished dosage form (e.g., tablet, capsule, or solution) that contains the drug (called the drug substance or active ingredient) in a particular strength, generally in association with one or more other ingredients. The FDA considers the different strengths and dosage forms of a drug as separate and distinct drug products.
[wp_ad_camp_1]
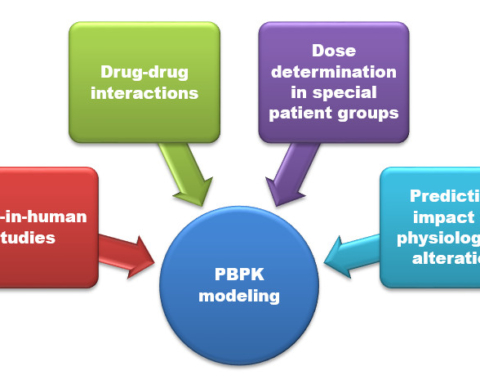
The process of new drug development and discovery is long, complex, and risky. Typically, it takes an average of 10 years for a drug to make it to pharmacy shelves after its first discovery. The major steps in this discovery, development, and approval process are summarized in the following figure