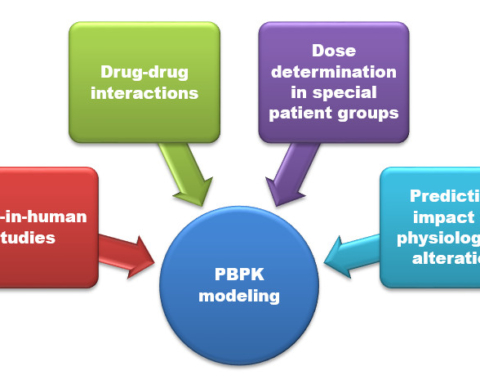
API – Active Pharmaceutical Ingredient
The U.S. Food and Drug Administration (FDA) is the government agency that regulates marketed drug products and approves marketing of new drug products. The FDA defines a drug product as a finished dosage form (e.g., tablet, capsule, or solution) that contains the drug (called the